An experimental drug being tested to see if it can treat Alzheimer’s disease helped slow the inevitable loss of clear thinking and memory that comes with the condition, researchers reported Wednesday.
It’s a rare success in a field littered with failures. No drug has yet been shown to reverse the symptoms of Alzheimer’s, which affects more than 5 million Americans and has no cure.
And this one is no home run. Everyone who took the drug continued to get worse. But those who got the highest doses got worse more slowly.
“We had a 47 percent reduction in decline at 18 months,” Dr. Lynn Kramer, chief medical officer of the neurology division of drug company Eisai, told reporters.
It was the second piece of good news on the Alzheimer’s front. Earlier Wednesday, researchers reported that lowering blood pressure to recommended levels was safe and reduced the rate of mild cognitive impairment — a precursor of dementia — by 20 percent.
Eisai and its U.S. partner Biogen presented results to an eager audience at the Alzheimer’s Association annual meeting in Chicago Wednesday.
They’ve been treating more than 800 patients with early-stage Alzheimer’s with various doses of the drug, called BAN2401. It’s a monoclonal antibody, a lab-engineered immune system protein designed to remove amyloid plaques from the brain.
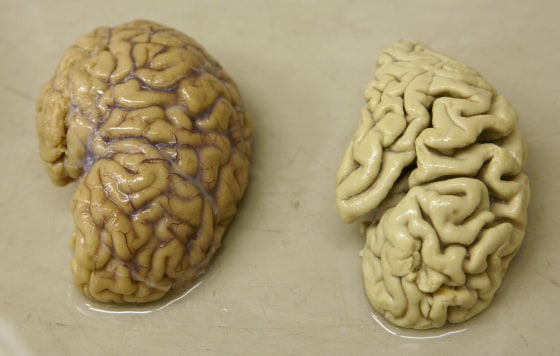
One leading theory about Alzheimer’s is that these amyloid clogs cause the disease.
“This is the first large trial to support the amyloid hypothesis,” Kramer said.
The drug cleared the plaques from the brains of the patients, by 93 percent at the highest dose.
Dr. James Hendrix, director of global science initiatives at the Alzheimer’s Association, says the findings are significant.
“Not only do we see a slowing in the rate of cognitive decline, we also see that reduction of amyloid burden in the brain as measured by amyloid PET (imaging)", Hendrix told NBC News. “That biomarker confirmation really adds confidence that what you are seeing in the primary measure is real.”
Still, the researchers had to tweak the results to be able to declare a success. When the trial was at the 12 month point, they said, they didn’t see a benefit. Now, after their patients have been infused with the drug for 18 months, they say they can see an effect.
To find out more, Hendrix said, the companies will have to design an advanced trial called a Phase 3 clinical trial — the final stage before seeking full Food and Drug Administration approval.
Dr. Howard Fillit, founding executive director of the Alzheimer’s Drug Discovery Foundation, supports the way Biogen and Eisai changed the study.
"The Biogen studies are being done in a different way than all of the studies we have done for the past 40 years," Fillit, who was not involved in the research, told NBC News.
"The Biogen study took advantage of everything that we have learned about doing modern, efficient clinical trials."
He called the results "amazing" — especially the reduction of amyloid in the brain.
It’s difficult to describe what a 47 percent decrease in in the worsening of symptoms looks like, researchers said. The patients all took a battery of tests used to diagnose and define Alzheimer’s.
“When you are talking about early-stage Alzheimer’s disease, you are talking about people who are living pretty well and still able to live independently at home,” Hendrix said.
“If you are able to slow that decline, that means they may be able to keep that quality of life for more time.”
One problem is a side effect that causes the brain to swell, which has been seen in all the monoclonal antibodies tried against Alzheimer’s. This affected about 10 percent of the patients.
“This is the second Alzheimer’s clinical trial that has demonstrated both clearance of amyloid from the brain and cognitive benefits — again, the studies were not large enough to definitely demonstrate cognitive efficacy and the BAN2401 study did not meet its primary endpoint," the Alzheimer’s Association said in a statement.
When trials don't meet their primary endpoints — when they don't do what they were designed to do — that can shed doubt on any results.
And depending on which test results were used, the improvement was either 30 percent or 47 percent - a discrepancy that troubles some experts.
But anything that might help would be welcome. “The global urgency to better treat and prevent Alzheimer’s disease and other dementias is higher than ever, and growing," the Alzheimer's Association said.
The group predicts that the number of people with Alzheimer’s will triple by 2050 unless someone finds a way to stop it.